Approach
Accurate Identification of "R" and "2B" States
We utilize pragmatic approaches to identifying "ground truth" to assess where you are today (R state). Once paired with management vision and organizational objectives to complete a road map, we are enabled to develop a comprehensive plan to help you get to where you need to be (2B state).
Each of us has countless hours in front of worldwide health authority inspectors. We know the trends and recent focus of inspections in both a pre-marketing and post-marking environments. We understand what they are really after and impart this knowledge to help your organization plan for success.
For remediation and functional builds, we employ strategic mapping methodologies garnered from Northwestern University's Kellogg School of Management and Lean Six Sigma tools.
But it is our approach to change management that sets us apart. This is often overlooked by executive teams or confused with simple communication plans. This is only a fraction of change management. We typically employ Prosci methods since they are simple, organized, and effect the most impactful behavioral change in the shortest period of time.
We utilize pragmatic approaches to identifying "ground truth" to assess where you are today (R state). Once paired with management vision and organizational objectives to complete a road map, we are enabled to develop a comprehensive plan to help you get to where you need to be (2B state).
Each of us has countless hours in front of worldwide health authority inspectors. We know the trends and recent focus of inspections in both a pre-marketing and post-marking environments. We understand what they are really after and impart this knowledge to help your organization plan for success.
For remediation and functional builds, we employ strategic mapping methodologies garnered from Northwestern University's Kellogg School of Management and Lean Six Sigma tools.
But it is our approach to change management that sets us apart. This is often overlooked by executive teams or confused with simple communication plans. This is only a fraction of change management. We typically employ Prosci methods since they are simple, organized, and effect the most impactful behavioral change in the shortest period of time.
Drug Safety Mind Set
Most development stage organizations do not yet possess a safety governance model, infrastructure to handle signal detection or worldwide reporting. We can reduce this risk and help you quickly establish a comprehensive PV system, efficiently and without sacrificing quality.
Most development stage organizations do not yet possess a safety governance model, infrastructure to handle signal detection or worldwide reporting. We can reduce this risk and help you quickly establish a comprehensive PV system, efficiently and without sacrificing quality.

Recent Work Snapshot
- Comprehensive PV Diagnostic Assessments and PV Functional Builds
- Global Regulatory and Quality Expertise
- Operational and Inspection-Readiness Preparations
- SOP Prioritization and Development
- Organizational and Governance Structure Design and Implementation
- Worldwide Drug Safety Office Strategic Planning, Launch & On-going Risk Management
- Setup and maintenance of the Pharmacovigilance System Master File (PSMF)
- PV Quality & Compliance enhancements including PSPs, REMS/RMP & Consumer Engagement
- Root Cause Analysis & CAPA Management
- Safety Vendor Management and Oversight
- Resource Planning including Business Process Off-Shoring/Outsourcing (BPO) & Insourcing
- Relevant Job Descriptions and Charter Development
- SDEA/PV Agreements
- Decision Support Tools, Dashboards and KPIs
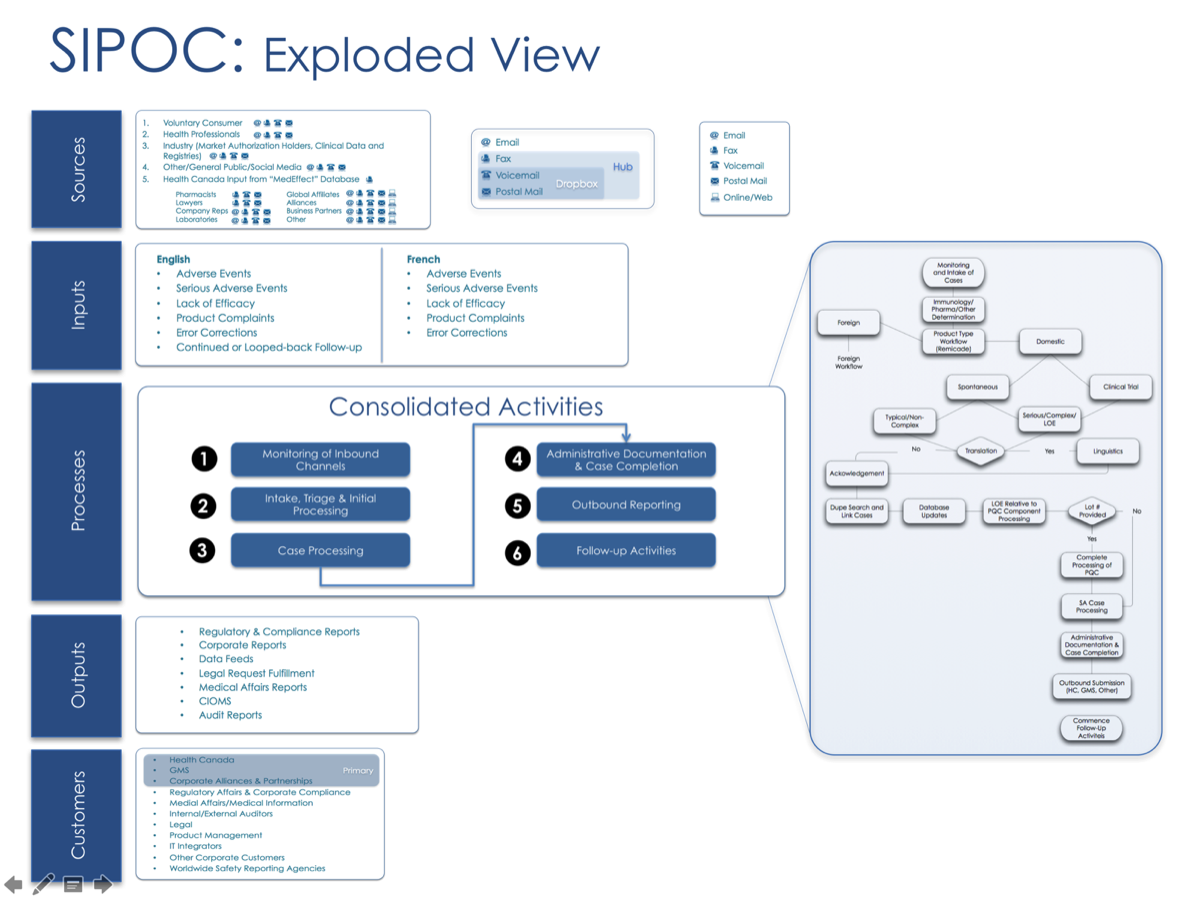
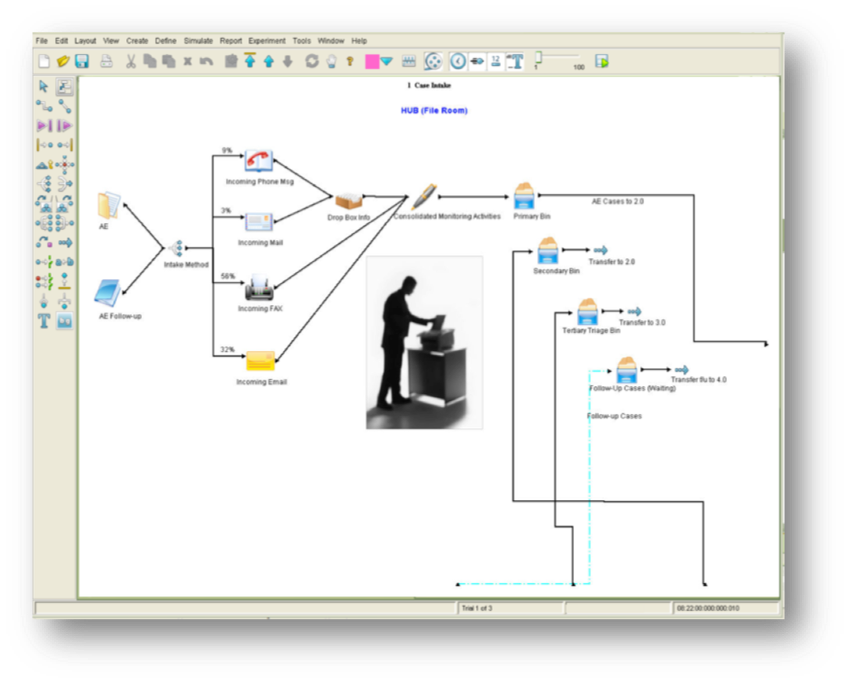
Process Simulators
Recent Duties and Accomplishments
- Global PV Lead at a mid-size biotech for the oversight of patient interactions from PSP activities and ensured appropriate PV provisions in all third-party agreements via SDEAs/PVAs.
- Conducted comprehensive PV diagnostic assessments, designed and implemented organizational and governance structures, advised on global SOP direction and development for several emerging BioPharma companies.
- Head of a Worldwide Drug Safety Office with oversight responsibilities for 65 DSOs and staff. Increased the overall quality of PV operations, morale, and competence of staff and vendors. Reduced global ICSR submission errors by more than 60% while improving on-time reporting to 98% on heavy PSP caseloads. Drove a 60% reduction in major and critical DSO/LSO CAPAs hitting the PSMF.
- Led the turnaround and recovery of an underperforming PV & PQC/PTC operation at a blue chip pharmaceutical company. Directed staff of 35 to improve the accuracy of case investigations and reporting. Reduced case aging from 7 days to 1; improvement in compliance went to 100% from 55%.
- Designed and delivered a comprehensive pharmacovigilance resource decision support tool to drug safety executives of a top 3 global pharma company to aid in determining and justifying appropriate staffing for EMEA, APAC, NA and LATAM Regions.
- Chaired joint QA/GPV leadership and integral participant on an Executive Steering Committee to address and remediate to multiple FDA 483 findings.
- Assumed responsibility for Worldwide Inspection Readiness efforts. Initiated global training programs and assembled proof books in advance of HA inspections resulting in significantly reduced findings.
- Accountable for the setup, oversight, timely updates and delivery of the Pharmacovigilance System Master File (PSMF) to the EUQPPV.
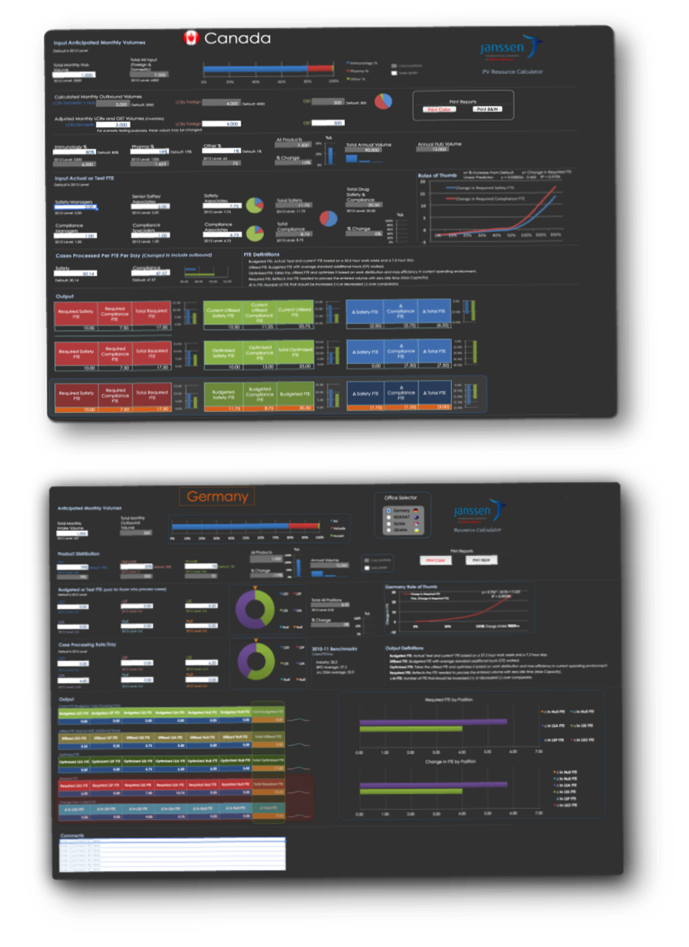
PV Resource Calculators