Who We Are …
Pharmacovigilance, Quality Assurance, Clinical Development, and Regulatory Affairs executives with expertise in
- Diagnostic Gap Assessments
- Inspection-Readiness Audits
- Operational Enhancements and Remediations
- Functional Stand-ups and Builds
- Client-side Program Management
We are former trained regulators, industry executives, coaches, clinicians, and elite consulting firm partners who know this industry and the governance required for compliance with worldwide regulations.

Advised Clients …
…Let Us Add You

What You Get …
Leadership
Changing or building new organizations, optimizing processes, and streamlining operations are rarely for the faint of heart. Combine that with the most heavily regulated industry on the planet, and the tasks appear insurmountable, even to the savviest executive teams.
We are experienced executives who have this expertise and who can help you through the maze of gap assessments, project, and change management challenges until success is achieved.
Changing or building new organizations, optimizing processes, and streamlining operations are rarely for the faint of heart. Combine that with the most heavily regulated industry on the planet, and the tasks appear insurmountable, even to the savviest executive teams.
We are experienced executives who have this expertise and who can help you through the maze of gap assessments, project, and change management challenges until success is achieved.
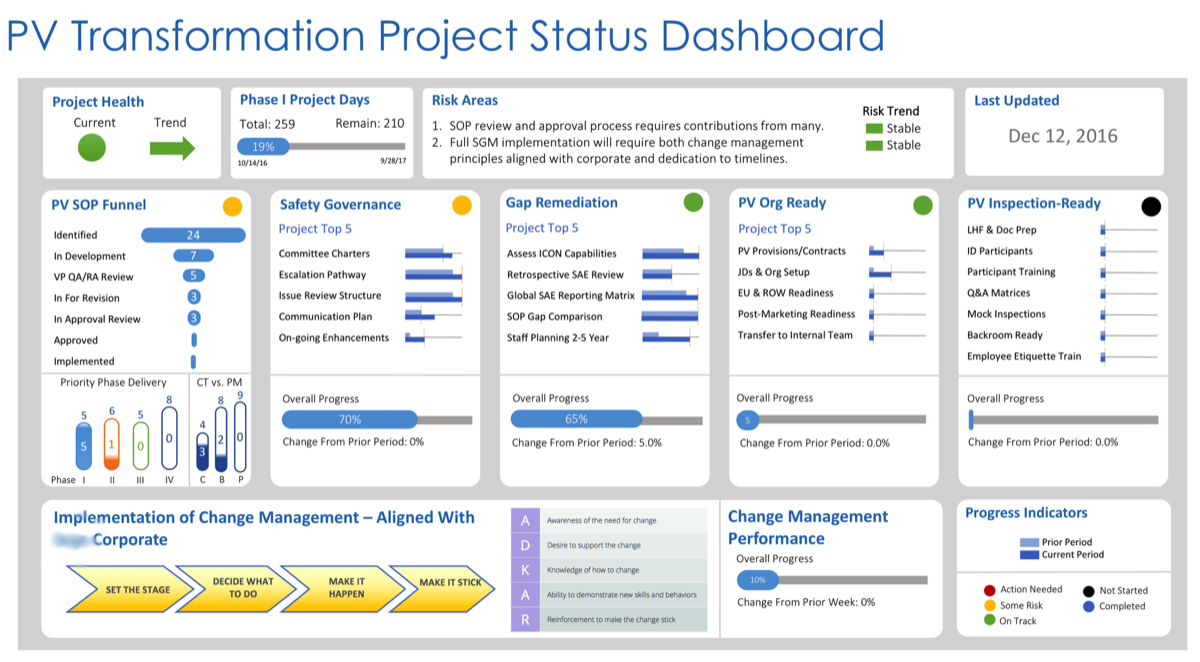
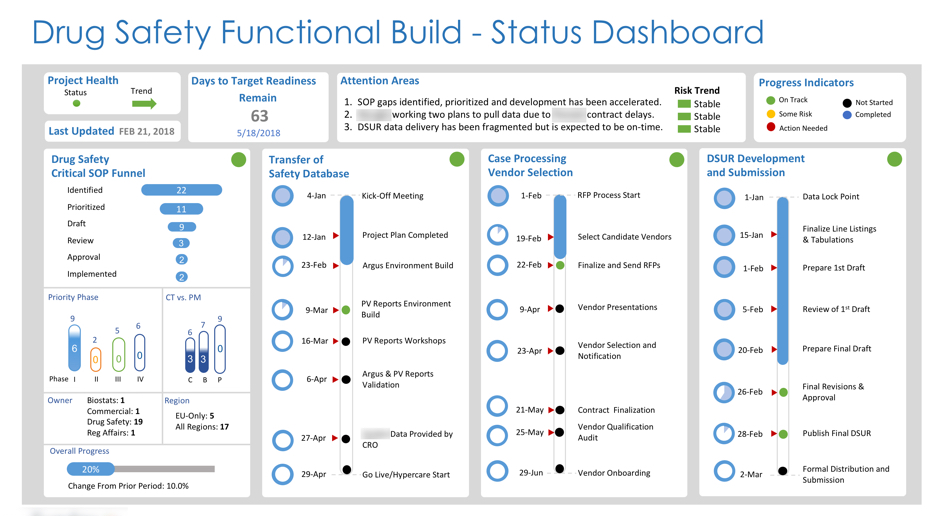
Functional Enhancements, Builds & Transformations
We are passionate about helping emerging and established biopharma organizations address a multitude of business challenges, including full functional stand-ups and transformations.
This includes the setup and management of pharmacovigilance systems, governance structures, supporting SOPs, quality and compliance enhancements, process and re-engineering improvements within clinical and post-marketing-stage biotech and pharmaceutical environments. The results yield operational and inspection-ready PV, Quality, and Clinical systems.
We are passionate about helping emerging and established biopharma organizations address a multitude of business challenges, including full functional stand-ups and transformations.
This includes the setup and management of pharmacovigilance systems, governance structures, supporting SOPs, quality and compliance enhancements, process and re-engineering improvements within clinical and post-marketing-stage biotech and pharmaceutical environments. The results yield operational and inspection-ready PV, Quality, and Clinical systems.
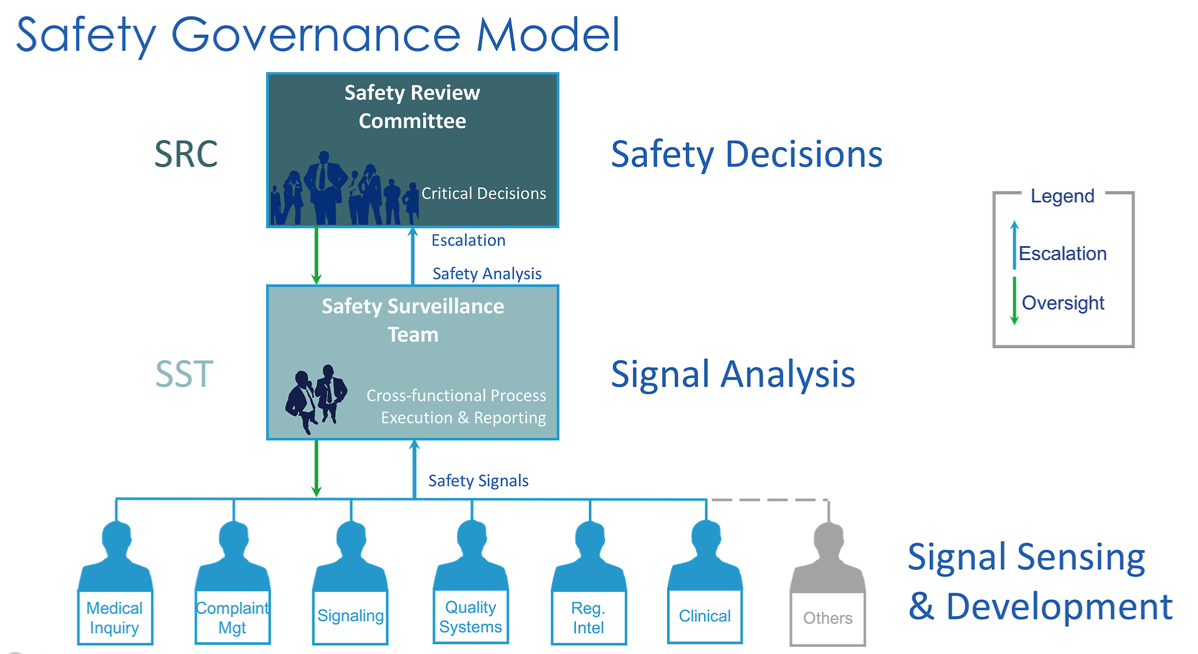
Trust
With whom you entrust your business enhancement will be an important part of your framework for success. Our global reputation and experience in making this journey efficient and even enjoyable set us miles apart from others. We would jump at the opportunity to work with you on your project.
With whom you entrust your business enhancement will be an important part of your framework for success. Our global reputation and experience in making this journey efficient and even enjoyable set us miles apart from others. We would jump at the opportunity to work with you on your project.



